
Hepatitis C
Jennifer Maclachlan, Benjamin Cowie, David Isaacs, Joshua S Davis
Recommendations
- Offer testing for hepatitis C (HCV) to people if they have:
- risk factors for HCV (see text)
- lived in a country with a high prevalence (>3%) of HCV (see table 5.1)
- an uncertain history of travel or risk factors.
- Initial testing is with anti-HCV test (HCV Ab). If this is positive, request an HCV RNA test.
- If positive, refer to a doctor accredited to treat HCV for further assessment.
Overview
In Australia, more than 230,000 people (over 1% of the population) are estimated to be living with chronic HCV.111 The majority of these people have a history of injection drug use. However, other priority populations include people who have been incarcerated; recipients of blood, tissues or organs before universal mandatory screening of blood donors in relevant countries; people with tattoos, skin piercings or those who have undergone other cultural practices involving skin penetration or scarring; and people born in countries with a high HCV prevalence. The National Hepatitis C Testing Policy recommends offering testing to all people in these groups.
People from refugee-like backgrounds should be offered screening if they have a history of any of these risk factors, and/or have ever lived in countries with a high prevalence of HCV. 29 If a person’s travel or risk history is uncertain, they should be offered screening for HCV. In a number of refugee- source countries, such as Syria, accurate prevalence information is not available, and there have been reports of disruption to screening or surveillance for blood-borne viruses. A number of Australian seroprevalence studies have demonstrated a higher prevalence of HCV in refugees, including in those from the Mekong region (3-8%109), Burma (2.8%48), and Sub-Saharan Africa (4%107), although other studies have reported a similar prevalence to the Australian population (e.g. 1% in selected African migrants108).
Chronic HCV is usually asymptomatic; however, if undiagnosed and unmanaged, it can cause advanced liver disease and/or liver cancer. HCV treatment is in a stage of rapid development, with new, highly effective and well-tolerated curative treatments becoming available, emphasising the need for early detection and proactive management. These treatments do not include interferon injections, and have minimal side effects.
| Table 5.1 Countries endemic for Hepatitis C29,40,43,48,112 | |||
| Country by region | Seroprevalence of HCV (%) | Country by region | Seroprevalence of HCV (%) |
| Angola | 5 | Kazakhstan | 3.2 |
| Armenia | 4 | Kuwait | 3.1 |
| Azerbaijan | 4 | Kyrgyzstan | 4 |
| Bolivia | 4.7 | Liberia | 3 |
| Burkina Faso | 5.2 | Malawi | 6.8 |
| Burundi | 11.3 | Mali | 3.3 |
| Cambodia | 4.1 | Mongolia | 10.7 |
| Cameroon | 13.8 | Mozambique | 3.2 |
| Chad | 5 | Niger | 3.2 |
| Congo | 5.5 | Pakistan | 5.9 |
| Cote D’Ivoire | 3.3 | Romania | 4.5 |
| Democratic Republic of Congo | 6.4 | Russia | 4.1 |
| Egypt | 14 | Rwanda | 4.9 |
| Estonia | 5 | Senegal | 3 |
| Gabon | 9.2 | Tajikistan | 4 |
| Georgia | 6.7 | Tanzania | 3.2 |
| Guinea | 5.5 | Togo | 3.3 |
| Guinea-Bissau | 4.7 | Trinidad &Tobago | 3.9 |
| Haiti | 4.4 | Turkmenistan | 4 |
| Indonesia | 3.9 | Uganda | 6.6 |
| Iraq | 3.2 | Ukraine | 4 |
| Italy | 3.2 | Uzbekistan | 6.5 |
Investigations
The initial diagnostic test for HCV is an anti-HCV antibody test. Except in neonates, who acquire maternal antibody passively, a positive result indicates prior exposure to HCV. Approximately 80% of people who are anti-HCV positive will have chronic HCV infection. Chronic HCV infection is confirmed by HCV RNA PCR testing. All tests should be performed with the informed consent of the individual, or their legal guardian where relevant (e.g. parents providing consent for children).
Figure 5.1: Hepatitis C testing and management algorithm
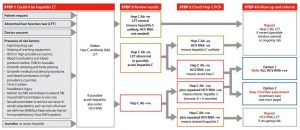
Source: Decision Making in Hepatitis C (ASHM resource)
Management and Referral
If HCV serology AND HCV PCR are both positive, the patient has confirmed HCV infection.
All those who have confirmed HCV infection should have:
- Counselling regarding the natural history, how to protect others from infection, the need for lifelong monitoring and the avoidance of hazardous alcohol consumption.
- A targeted history and physical examination, looking for symptoms or signs of chronic liver disease.
- Baseline LFTs, FBE, iron studies, UEC and upper abdominal ultrasound.
- HCV genotype testing (this is Medicare-rebatable and can be requested by any doctor, not only specialists).
- Serology for HAV, HBV (HBsAg, HBsAb and HBcAb), Hepatitis delta virus (HDV) and HIV if not already done.
- A repeat consultation, once these results are available, to decide on a management plan and whether specialist referral is needed.
- The following patients should be referred to a specialist (ID physician, gastroenterologist or GP with accreditation to prescribe HCV medications):
- proven or suspected cirrhosis
- suspected extra-hepatic manifestations (e.g. glomerulonephritis, lichen planus, porphyria cutanea tarda)
- the patient is ready to consider being treated for their HCV
- co-infection with HBV or HIV.
- The following patients with chronic HCV infection should be offered surveillance for hepatocellular carcinoma, with 6 monthly ultrasound and serum AFP:
- anyone with proven or suspected cirrhosis
- anyone with a family history of HCC in a first or second-degree relative.
- People living with hepatitis C should be vaccinated against hepatitis B if they are susceptible to HBV infection (HBV vaccine is funded for this indication in many jurisdictions), and they should be offered vaccination against hepatitis A virus (HAV) if they are susceptible to HAV.
From 2016, treatment for most people with HCV involves 12 weeks of all-oral combination therapy, with few adverse effects and a >90% chance of cure. Such therapy is available to those with access to Medicare, via the PBS from 1/3/2016.
Considerations in Pregnancy and Breastfeeding
The risk of HCV transmission from mother to child is approximately 3-5%. Neither the mode of delivery nor breastfeeding appears to affect this risk of HCV transmission. Note: HCV testing is not part of routine antenatal screening.
Current treatments for HCV infection are either known to be unsafe or have not been adequately tested to determine safety in pregnancy and while breastfeeding.
If a woman is diagnosed with HCV infection antenatally it is important to ensure the obstetric services are aware of her diagnosis. Seek specialist advice where concerned, and arrange ongoing follow up and management after delivery. Unnecessary instrumentation (such as fetal scalp electrodes and instrumental deliveries) should be avoided. Higher risk of mother-to-child transmission of HCV has been reported in the setting of maternal HIV co-infection.
Considerations for Children
Children born to mothers living with HCV infection should be tested at 12-18 months of age for anti-HCV antibody, to determine if they have acquired infection, with subsequent HCV RNA testing in children who are antibody positive to assess for chronic infection. Children with HCV infection should be referred to a paediatric viral hepatitis specialist for ongoing management.




